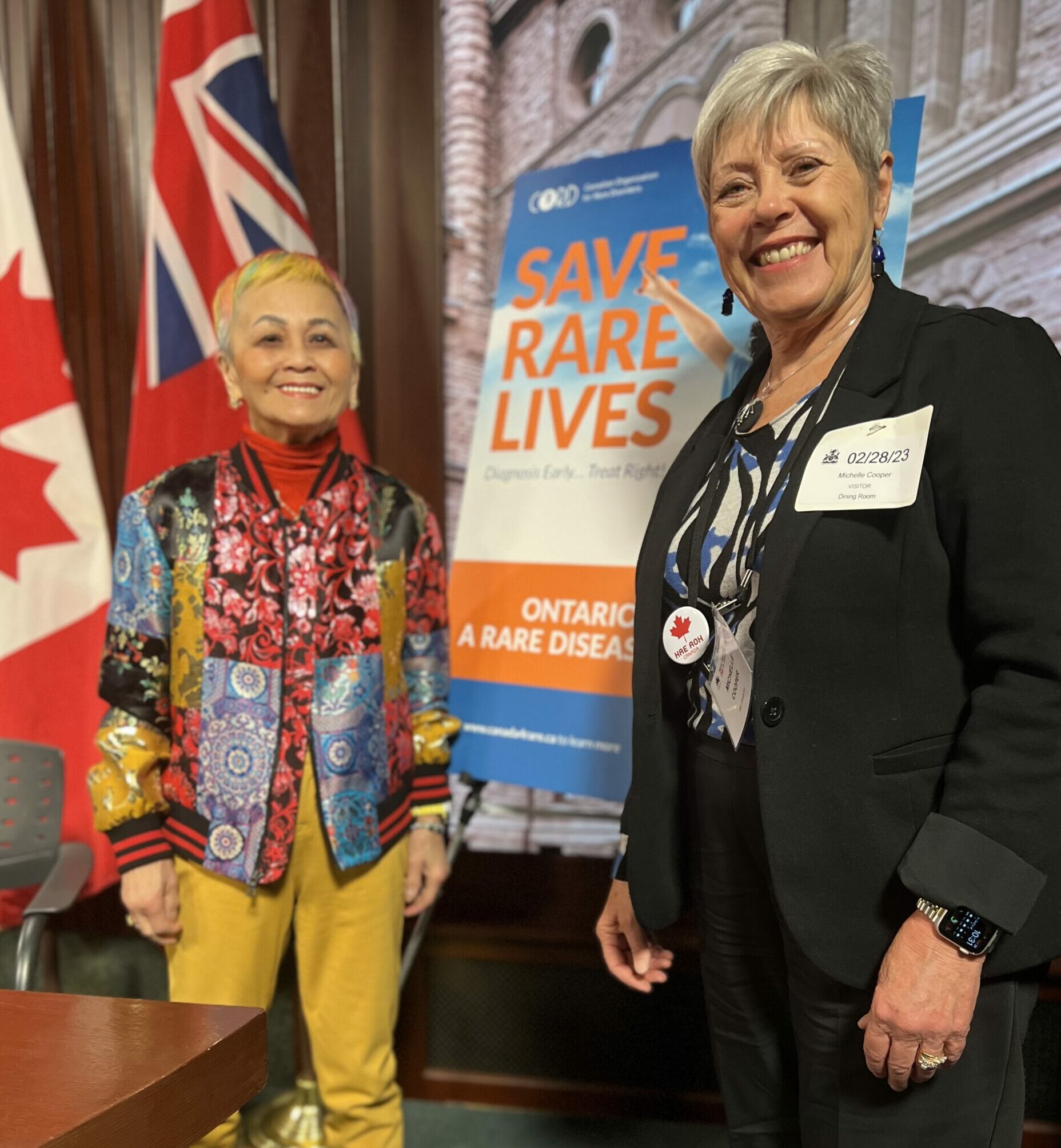Help raise HAE awareness and have fun at the same time!
Between now and the end of May, we are encouraging everyone to join the 2024 HAEi Activity Challenge. After each time you participate in a wellness activity (physical or otherwise) you simply add it to your profile and watch Canada’s steps grow!
Latest News
Rare Disease Day Breakfast with CORD
To honour Rare Disease Day 2023, HAE Canada President, and Ontario Regional Director, Michelle Cooper,
HAEC Board member featured in Canada’s Rare Voices 2023
For Canada's Rare Voices 2023, Patient Voice teamed up with 12 patients and their families,
Updates to our Board of Directors
We have some announcements to make about the HAE Canada Board of Directors. The 2022-2023
2022 HAEi Global Leadership Workshop
Traditionally, every two years, HAE International (HAEi) hosted the HAE Global Conference where patients, caregivers,
CBS Announces Results of the RFP for PPRP
Canadian Blood Services (CBS) Announces the Results of the Request for Proposals (RFP) for Plasma-Derived
The 2022 Virtual Global Walk
To celebrate hae day 🙂 over 5,000 people around the world contributed their steps to the
Health Canada Approves ORLADEYO
We are thrilled to share BioCryst Pharmaceutical's big announcement: Health Canada has approved their oral,
Takeda Canada Awarded Tender for TAKHZYRO
Héma-Québec, the organization in Quebec that manages blood and biological products, has awarded a
HAEi Advocacy Academy
HAE International is offering free, online advocacy courses. This extremely comprehensive program is designed to support people with
Our Next Patient Information Update: November 20, 2021
Please join us on Saturday November 20th from 1:00-3:00pm ET for our next Update! Details
Support Family Testing
Give valuable insights in HAE science
Hereditary Angioedema (HAE) is a hereditary disorder. It is therefore very important to have other family members tested. To help you in this process HAE International has developed two documents for patients. You can download the documents below: